About Biosimilars
What is a Biosimilar?
A biosimilar is a biologic medicine that is highly similar to the reference product notwithstanding minor differences in clinically inactive components. According to the US Food and Drug Administration (FDA), biosimilarity means there are no clinically meaningful differences between the biological product and the FDA-approved reference product in terms of the safety, purity, and potency.


How are Biosimilar diffrent from other generic medicine
Amneal Pharmaceuticals, Inc. (NYSE: AMRX) is an integrated specialty pharmaceutical company powered by a robust U.S. generics business and a growing branded business as well as deepening portfolios in.
How are Biosimilars different from other generic medicines?

Antibiotic
Small Chemical Molecule


Calcitonin
Simple biologic


Filgrastim
Minor complex biologic


Erythropoietin
Moderate complex biologic


mAb
Complex biologic

Biologics are far more structurally complex than small molecule pharmaceuticals such as antibiotics.
The value of biosimilars

Biosimilars are projected to save an estimated $38.4 billion from 2021 to 20251

Biosimilars are approved according to the US FDA’s rigorous standards

Lower costs also mean greater accessibility for patients who need it most

Their safety is continually monitored through post-marketing surveillance
-
Projected US Savings From Biosimilars, 2021–2025. Published in: American Journal of Managed Care, Volume 28, No. 7 (July 2022) Posted on RAND.org on January 26, 2022 (https://www.rand.org/pubs/external_publications/EP68829.html)
Messaging Headline Goes here. lorem ipsum dolor

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.

Headline reflacting benifits
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old.
Approval Pathway2
Biosimilar medicines undergo a rigorous regulatory approval process to ensure efficacy, safety and quality. Since safety and efficacy have already been established for the reference biologic, the goal of the biosimilar development program is not to require additional large clinical studies, but rather to demonstrate that the compound is highly similar to the reference product with no clinically meaningful differences. It does this using a stepwise approach outlined in the Biologics Price Competition and Innovation Act (BPCI Act). A biosimilar product application must include data demonstrating biosimilarity to the reference product. This usually includes data from:
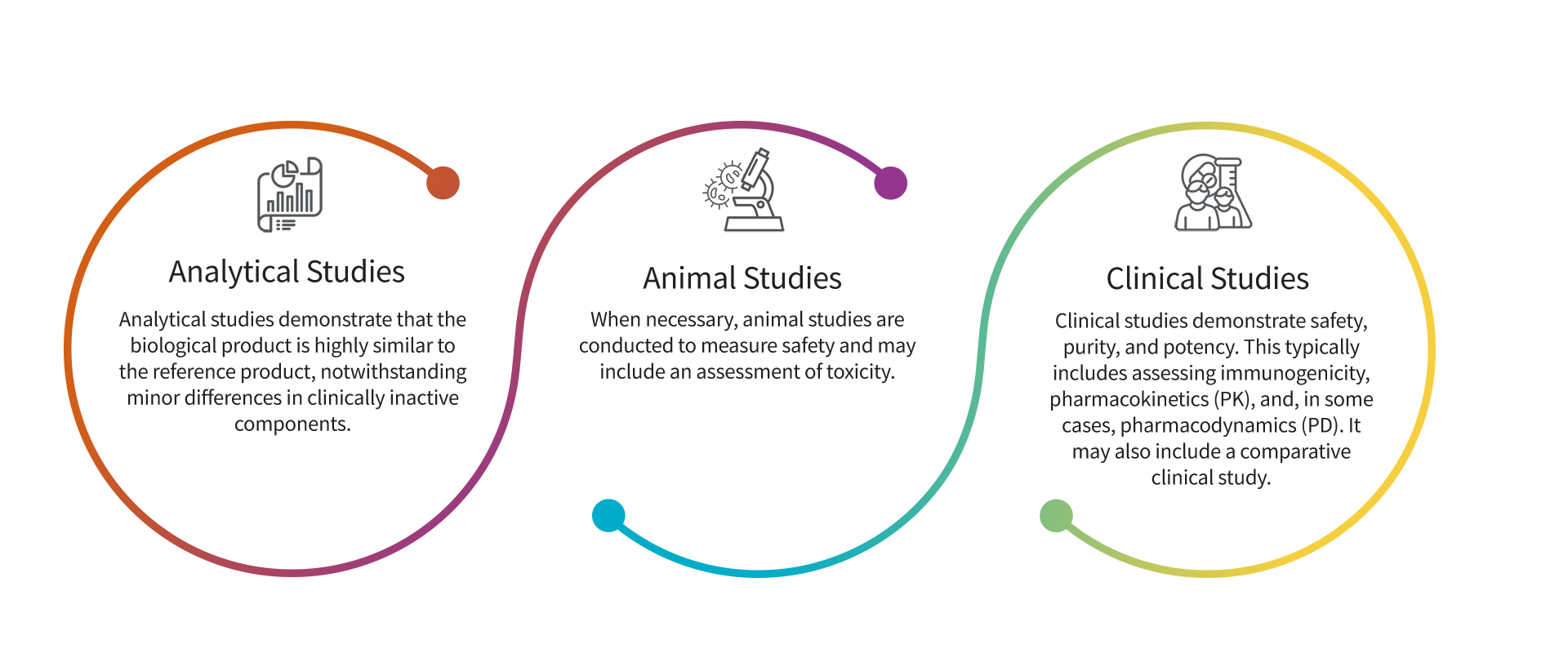

Learn more about the US FDA approval process at FDA.gov.
2. U.S. Food & Drug Administration website accessed 8/1/2022 (https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval ) (https://www.fda.gov/media/108621/download )