Amneal
Biosimilars
Increasing access while
lowering cost
The value of biosimilars

Biosimilars are projected to save
an estimated
$38.4 billion from 2021 to 20251

Lower costs mean greater access
for appropriate patients

Greater access
may mean improved
health outcomes

Reducing the cost of
medicine helps
to create a
more sustainable healthcare system
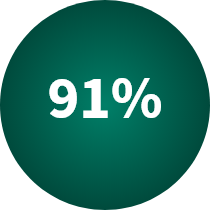
In 2022:
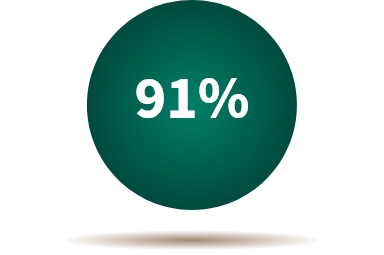
of the prescriptions dispensed in the United States were biosimilars and generics, due to their low cost and high value to patients.

was saved by patients in
the United States* from using Amneal’s generic
and biosimilars medicines.
*Amneal’s generic savings in the United States in 2022 was calculated by taking the total national savings estimated by the Association for Accessible Medicines and determining Amneal’s market share by volume, data of which was derived from IQVIA.
What is a biosimilar?
A biosimilar is a therapeutic product that the FDA has determined to be highly similar to an approved biologic, known as a reference product, and has no clinically meaningful differences in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowed.
Biosimilars are created in living cells and require significant expertise and state-of-the-art technology in development and manufacturing.
Our three FDA-approved biosimilars are:
ALYMSYS® is a registered trademark of mAbxience Research, SL.
FYLNETRA® and RELEUKO® are registered trademarks of Kashiv BioSciences, LLC.
Which products are of interest to you? Let us know.
Connect with us

The biosimilars market is evolving fast. And we’re evolving just as fast to stay ahead of market needs.
— Courtney Koster
Sr. Director, National Corporate Accounts
Why are biosimilars so important?
Hear the dynamic Amneal team describe the value of biosimilars.

Reference: 1. Mulcahy A, Buttorff C, Finegold K, et al. Projected US savings from biosimilars, 2021-2025. Am J Manag Care. 2022;28(7):329-335.